價格:免費
更新日期:2020-06-01
檔案大小:1.3 MB
目前版本:2.2
版本需求:系統需求:iOS 13.4 或以後版本。相容裝置:iPhone、iPad、iPod touch。
支援語言:英語
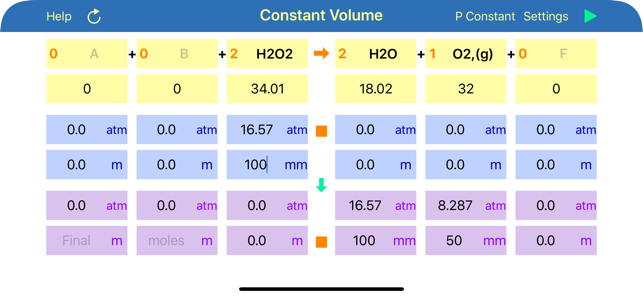
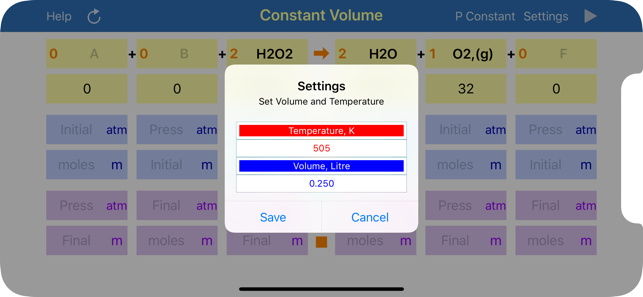
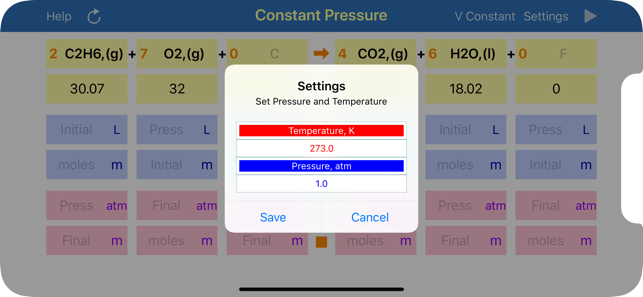
Gas Phase Stoichiometry is an ultimate tool for parameterisation of unidirectional chemical reactions that occur in a gas phase. The application balances chemical reactions, derives molar masses, moles, pressures and volumes of reactants and products based on their chemical formulas, stoichiometric coefficients and user defined temperature, enclosing volume or external pressure. Application provides two layouts: the first is for reactions occurring at the user defined constant volume, where moles and partial pressures of compounds are calculated and the second is for reactions occurring at the user defined constant external pressure, where moles and volumes of gaseous compounds are analysed.
User is required to provide initial or final (after reaction accomplishment) pressures (volumes) or moles of any of the reaction component, to calculate all required and obtained pressures (volumes) and moles for all components. If data is available for several components, then limiting reagent will be found based on reaction stoichiometry. Either mole or pressure (volume) should be filled in for each compound, but if both are provided and they do not match, then moles value will have a preference and the stoichiometrically matching pressure (volume) will be recalculated.
The app can get data directly from Stoichiometry application for calculations of pressures/volumes of gaseous compounds. The data may include balanced chemical reaction, molar weights and initial mole quantities.
For a general unidirectional chemical reaction:
aA + bB -> cC + dD,
where A, B, C, D – are compound formulas and a, b, c, d are stoichiometric coefficients, the components will only react in a defined ratios. Thus, every “a” moles of compound “A” will be needed “b” moles of compound “B”, to produce “c” moles of “C”. If more “B” will be added than it is required, according to the reaction stoichiometry, then all spare amount of “B” will be left unconsumed. In such a case compound “A” will be called a “Limiting Reagent”, as its “small” amount limits or stops reaction before all other reagents are consumed.
While mathematics behind the stoichiometry is rather simple and straightforward, the continuous need to convert moles obtained from balancing chemical reaction to actual pressures or volumes, routinely casts burden upon students and lab workers. In order to facilitate and significantly accelerate the process, the “Stoichiometry Gas Phase” application has been developed.
Please look for working examples at www.volard.wordpress.com.
Important Points:
Either moles or masses can be filled in, but if by mistake both are provided and they don’t match, then the moles value will have a preference.
If one of the compounds is not in a gas phase, then only its mole values should be considered.
Component will be taken into consideration once the stoichiometric coefficient is provided for it.
App uses ideal gas equation to convert moles to pressure or volumes and backwards.
App assigns dot as a decimal point.

支援平台:iPhone, iPad
